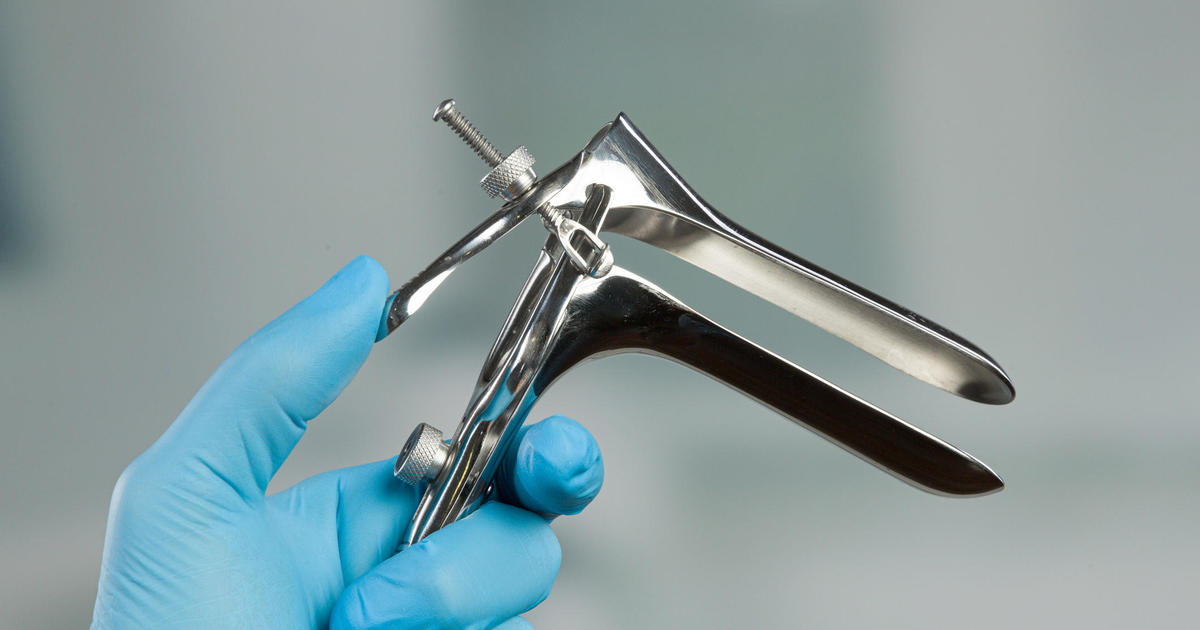
Alternatives to the often dreaded pap smear may be on the horizon for cervical cancer screenings.
Typically, the procedure involves inserting a device called a speculum, which helps open the vaginal canal, in order for a swab to be inserted and brushed against the cervix to collect a cell sample. All this is done while laying back with feet hoisted in stirrups to keep legs spread apart.
For some, pap smears are an uncomfortable but necessary evil when it comes to health screenings. For others, it’s an experience bad enough to avoid a doctor’s visit, risking not detecting cancer cells early.
But this year, some health care companies are preparing to introduce self-collection options, allowing patients to skip awkward interfaces with healthcare professionals.
Labs are now able to test samples from the vaginal walls, as opposed to from the cervix itself, which has been a key change in making self-collection options possible, according to a report from the New York Times.
Have self-collection tests been FDA approved?
In May, the Food and Drug Administration approved primary human papillomavirus self-collection for cervical cancer screening in a health-care setting, allowing for a more convenient and private option — a move the American Cancer Society applauded for expanding access to screening and reducing barriers.
“Despite the benefits of cervical cancer screening, not all women and people with a cervix get screened regularly,” Dr. William Dahut, chief scientific officer at the society, said in a news release at the time. “Most cervical cancers are found in people who have never had a cervical cancer screening test or who have not had one recently. That’s why adding self-collection in a health-care center as a screening method for this potentially deadly disease can make a huge impact.”
Tests included in the self-collection approval included Onclarity HPV, made by Becton, Dickinson and Company, and cobas HPV, made by Roche Molecular Systems.
But researchers are looking to take things one step further, seeking approval for at-home self-collection tests.
After reviewing midpoint clinical trial data, for example, the FDA granted company Teal Health priority status when submitting its final study data on the company’s “Teal Wand” at-home collection device for FDA review.
While screening at home is already available in other countries, the NYT reports this method could gain approval in the United States by early next year.